| |
| |
|
| ARCHIVE COPY - Abridged 2003 |
UP to TOP DOWN |
 Musical Instruments - The steeldrums
Musical Instruments - The steeldrumsABSTRACT
FOR more than eighty years gas-nitriding has been applied to steel to get better wear resistance, fatigue strength and corrosion protection. PANArt Ltd are one of the first to apply this treatment to sheet metal [to produce a new gradient composite material specifically for steeldrum instrument manufacture]. The result are instruments with [improved] durability [where the new foundation material] offers better [inherent corrosion protection,] and [the desirable qualities] of higher [than usual] tensile strength and yield point.
1. Introduction
Nitriding procedures are thermo-chemical processes, where the surface of the steel is enriched with nitrogen. Treatments normally occur at temperature of between 500°C and 590°C in a medium which can give off nitrogen. Such media are for example gas, plasma, saltbath or of powdered form. In our case, a gas media is used in the process called gas-nitriding.
Normally nitriding is applied to special tools and elements of machines and cars. It is rare to find gas-nitriding in use with sheet metal, except in a few cases; but it can be found in use, in particular by the spring industries.
PANArt Ltd has performed experiments with different surface treatments [for steel] in search of a stronger material. Nitriding in a saltbath has been used for the Blackpans; here [the active elements] [] are not only nitrogen, but also carbon. The duration of this process is [relatively fast] between 10 to 40 minutes, and the [resultant] surfaces get very hard. Gas-nitriding is a longer duration process, but it can be better controlled. The effect of gas-nitriding is to improve wear resistance, fatigue strength, corrosion protection and the qualities of the steel used in [steeldrum] musical instrument [manufacture].2. Nitrogen and Nitride Formers
Nitrogen forms a chemical bond with [among others,] the elements aluminium [Al], magnesium [Mg], silicon [Si], titanium [Ti], vanadium [V], chromium [Cr], molybdenum [Mo] and iron [Fe]; to produce nitrides. These elements [when alloyed with; or remain residual traces in iron [Fe] steels] are called nitride formers. Nitrides are hard elements, and [act] similarly to the familiar carbides [compounds of carbon] precipitated into the iron matrix [as associated with case-hardening using carbon].
The radius of atomic nitrogen is 50% of the radius of atomic iron; and about 8% smaller than atomic carbon. Therefore the inclusion of atomic nitrogen into the interstitial position of the iron lattice is possible. [This behaviour is classified in Physical Chemistry as being of the non-stoichiometric compounds of the interstitial carbides [MxC], nitrides [MxN] and hydrides [MxH]; where the respective atoms may occupy some, if not all, of the small spaces between the larger metal atoms.]
For tools and elements of machines and cars; gas-nitriding is normally applied to alloyed steel to get a high hardness (to 1200 Vickers*). For [steeldrum] instruments this hardness is too high; the steel can not easily be deformed, and it breaks. But [for our purposes and requirements, we start with] deepdrawn steel (Fe PO4 / St 14.03), having a very low content of nitride formers; the hardness [we seek to control] will then not increase too much.
[ * Vickers Hardness = HV ]3. The Process
The forming of nitrides is a chemical reaction between nitrogen and iron, [this chemistry is not easily achieved however]. []
To work at normal [atmospheric] pressures, ammonia gas [] is used [to produce the required nitrogen]. [The initial stage of gas-nitriding of iron using ammonia, is virtually the reverse of an ammonia Haber production process where; with iron as a catalyst, undertaken at similar temperatures - but here without the high pressure - the first stage of this reversible reaction now proceeds instead to the alternate equilibrium; to dissociate the ammonia into its constituent gasses - nitrogen and hydrogen.]
[At standard temperatures and pressures (stp), iron is inert to nitrogen; or to its absorption. The diatomic molecule of nitrogen is anyway too large to penetrate the surface; and even if it were a single atom, it would not have enough kinetic energy to penetrate into the fields of the iron lattice matrix.
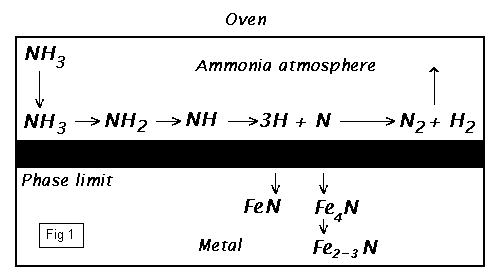 |
2NH3 ----> 3H2 + N2 |
IN an oven; a current of ammonia gas is passed over the work pieces which are heated slowly to 580°C. The ammonia, splits under catalytic [Fe] interaction on the glowing surface, dissociates into atomic nitrogen and atomic hydrogen (Fig.1). Only the atomic nitrogen penetrates into the metal surface. However, the side reaction of converting atomic nitrogen into molecular nitrogen is faster than the absorption of nitrogen into the hot iron surface. [To proceed however], the partial pressure of the nitrogen-atmosphere on the iron surface must be [kept relatively] high. In order to maintain an advantageous partial pressure of atomic nitrogen; a strong current of ammonia must be continually replaced and circulated around the work.
[At the appropriate temperature, activity begins. The precise chemistry is a little unclear, but the results are demonstrable. Either through direct chemical action with iron, or assisted by secondary catalytic processes, the active monatomic] nitrogen enters the surface matrix of the metal. [The nitrogen then proceeds to diffuse randomly within the lattice, towards the centre of the material. In this state - the iron (alpha)-a-lattice remains in body centred cubic (bcc) form up to 906°C - the nitrogen in the centre of the cubic iron matrix, is classed as the interstitial nitride [Fe4N]; despite its classification, the nitrogen can migrate and diffuse within the material.]
[Conditions are different around the surface however. The chemistry, still a little unclear; but again either through direct chemical action, or by secondary catalytic processes, the iron atoms at the surface become scavenged and break away. The lattice structure begins to break up and opens. In this area, but only for a very short distance into the material, with the ionic bonding of the (bcc) lattice structure broken; it is believed that the stoichiometric ferrous [Fe3N2] and ferric [FeN] nitrides form and collect. Within this area too, some percentage will be of the interstitial [Fe4N] type.]
[Throughout the process duration, the surface area sees high activity; allowing a steady flow of nitrogen into the body of the material, and experiencing the in and out flux of atoms that are not finally reacted or captured.] Near surface nitrides conglomerate into small crystals, [further stressing the surface lattice.] This is the compound layer. [The layer slowly deepens as the process continues].
The layer of material beneath, and the main body of the material, is called the diffusion layer. []
The duration of the process can be varied between 5 to 100 hours. Afterwards, the oven is slowly cooled down.
_f2_re_tuniversity_panart.gif) |
4. The Sandwich
This nitrided steel is now of a new structure, and may therefore be considered as a new material. It can be called a gradient composite material; it is not metallic anymore. The new structure is a sandwich with an hard surfaces and soft core. The nitrided steel has an hard compound layer on both sides. The core is the diffusion layer, which is less hard.
A micro-graph of the compound layer of nitrided sheet metal (Fe PO1), after nine hours (9h) of gas-nitriding, is shown in Fig.2. The thickness of the compound layer is about 15µm. Within the compound layer are pores. The hardness of the compound layer is 400 HV.
_f3.gif) |
In Fig.3 the diffusion layer is shown with the [interstitial] nitrides in the form of needles. This diffusion core has a hardness of between 157 HV and 177 HV. The original hardness of the sheet metal (Fe PO1) was 86 HV.
The duration of the gas-nitriding process and the composition of the [original] material, influence the parameters of the hardness.
5. The Properties
The increase of hardness is only a part of the changes after nitriding. The gas-nitriding improves the mechanical properties of the work pieces; particularly the wear resistance and fatigue strength. The tensile strength and the yield point of the material have also been increased. The compound layer is stable to temperatures of about 500°C.
In the surface arise high compression stresses. The surface is no longer metallic and has good corrosion resistance.
6. The Problems
It is a special case to make [steeldrum] instruments with nitrided sheet steel. [Because of its stiffness,] nitrided sheet steel is not meant to be deformed. [For our purposes] nitriding can only take place after 'sinking'; this is why the process is divided into two steps: In the first, the steel is deepdrawn; in the second, it is nitrided.
As tuning is performed after nitriding, a degree of ductility is needed; further, the steel should not be so hard as to break.
Previously where [drum-head] steel has been deformed by hand; the unequal areas of thickness caused unequal areas of hardness over the work area of the material. [Deepdrawn sheet addressed this problem by allowing a material of uniform thickness to result.]7. The Parameters
Nitrided sheet steel parameters such as hardness and thickness of both the compound layers, and the diffusion layer, are mainly influenced by the composition of the steel. PANArt has worked with several sheet metals of different composition. This requires the knowledge of the composition of the material. Alloyed Steel ZStE for example, gets very hard with nitriding because of the nitride formers such as silicone [Si] and titanium [Ti] contained in the steel. So the process duration has to be adjusted.
Nowadays we work with common steel (Fe PO1 or Fe PO4), which is used for deepdrawing. This steel has a very low content of nitride formers.
The nitriding process duration is between 7 and 12 hours; left any longer - say 2 hours, and the hardened steel would break with further deformation. So the final parameters are strongly influenced by the duration of the nitriding process.
With the correct selection of steel composition, and setting of the nitriding process duration, the properties of the sheet material can be adjusted to our needs.
It has been a long path, of trial and error, to find the right parameters for steel with which to make [steeldrum] instruments.
8. Stress+Strain Diagram
 |
_f5.gif) |
The comparison leads to the following observations:
- The tensile strength increases from 300N/mm2 to 500N/mm2 (MPa)
- The yield point increases more than twice from 160N/mm2 to 378N/mm2
- The modulus of elasticity increases from 190kN/mm2 to 200kN/mm2 (GPa)
- The uniform elongation decreases from 26% to 9%
9. Treatments after Nitriding**
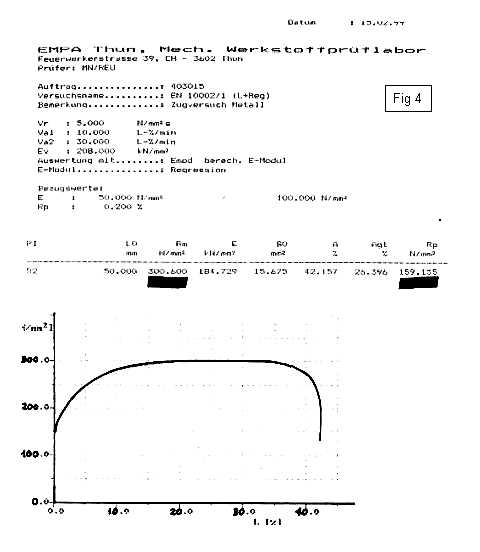
After forming the note shells, the instruments are heated to release macro-stresses. Fig.6 shows a stress-strain-diagram of nitrided steel, which has been heated for 4 minutes to 500°C. The E-modulus, the yield point and the tensile strength again increase. This shows that the properties are again improved.
It is not understood why this happens, probably because of a shocking in air, so the nitrides age and brace the iron matrix.
 |
- Heated for 4 minutes at 500°C - Used in Pang Manufacture |
[With a view to a presentable surface finish,] chrome and nickel do not hold to the non-metallic compound surface layer of the nitrided steel. The instruments can be polished however, so that they look like having been chromed. With polishing only the pores go away, the compound layer still exists.
It is also possible to close the pores with oil, or wax; where the results are the colours of oxidation.
The surface offers good corrosion resistance.
10. Conclusions - With Reference to the Instrument Maker**
The hard surface and the pliable core unites several important qualities:
The steel can still be elongated by 8-10%; the material is pliable enough to form the shells. In the hard surface breaks can arise, the core however remains ductile.
The yield point is high, but the steel can still be worked by hand. The influence of the direction of sheet-rolling [on the grain structure] is reduced because the nitriding homogenises the structure.
The high yield point allows stable shells to be made which produce, together with a good geometry, stable sounds. Only impacts which are stronger than the yield point may cause plastic deformation.
Nitriding produces high compression stresses in the compound layer. These stresses do not go away, the compound layer is stable to 500°C, the stresses are internal. Changes of curvature will quickly change the frequencies because of the high stresses in the compound layer. Tuners call that a good tuning force of the material. It is mainly in the surface where the stresses are achieved.
As the material is strong, the tuner is more at liberty to [apply his] tuning skills; he is [not inhibited] by having to adapt to the material [each time he tunes a new instrument]. Different tuning [choices] are possible because the spread of the partials happens quickly.
With [selected note] shaping, the character of the sound can be influenced.
The problem of weakening the E-modulus is also solved. An E-modulus of 200GPa provides for fast bending wave and therefore to good sound radiation.
It is perhaps possible that Poisson's ratio is changed with nitriding.
The work hardening is eliminated [annealed] with nitriding. But after nitriding the steel is quickly strength hardened.
The nitrided steel has less damping than common steel, but is still good enough to make percussive instruments. It is the soft core which provides a certain damping.
11. Conclusions - General
Hardening sheet steel by gas-nitriding offers new possibilities in making sheet material stronger. It is a [relatively] easy treatment with low costs.
The processes can be [generally] divided into two steps; first the [deepdrawn] forming, and second the hardening by gas-nitriding.
The material does not need to be strengthened with work hardening, [as was traditional with 'sinking'. Deepdrawn sheet] - the rawform hemisphere - [addressed part of this problem, and additionally allows a material of uniform thickness to result.]
The tuner becomes independent from the steel producers, and no longer needs to search for special composition steels.
The gas-nitrided sheet steel has a new structure, and the sandwich unites several [desirable] qualities.
The new structure is a sandwich with an hard surfaces and soft core; it is [near] brittle and at the same time ductile.
The new structure has a new combination of characteristics which does justice to the complexity of the instruments [manufacture and tone]. Remember the string? It has a strong core around which is wound another soft material.
Although instruments like steelpans can be built [using] this new material; a consequence of this new material is the development of new note-shapes, and of new sounds.
It has been a challenge to invent new [materials and] instruments and PANArt are sure it will enrich the future [of] music.
12. Acknowledgements
The authors wish to thank Prof. Rufer, HTA Biel CH; B. Stucker, Härterei Duap, Herzogenbuchsee CH and H. Schaub, Härterei Hans Schaub AG, Fällanden, CH.13. References
1. H. Maeder and J. Bartanus, Diplomarbeit 1999, Werkstoffkunde HTA Biel, CH.
2. Merkblatt Stahl 447, Nitrieren und Nitrocarburieren,1983 Düsseldorf, D.============
** NOTE: Editorial placement changes:
The original order of position of headings/subjects of Sections numbering 9 (was 10) and 10 (was 9) have been interchanged here.
eEd.
============
|
| PANArt AG Engehaldenstr. 134 CH-3012 Bern Switzerland http://www.hang.ch info@hang.ch Tel: + 41 (0)31 301 3332 |
|
||||||

| [2nd Ed] © 2003: tobagojo@gmail.com - 20031029 - 1m20071228 - 2m20140615 Historic Update: 16 November 2003; Last Update: 29 June 2014 02:11:00 TT Processed by: JdeB - Islands Research |
UP |